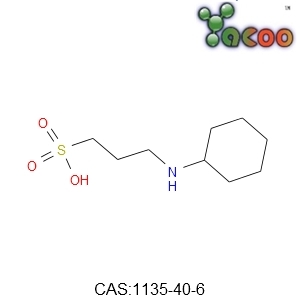
There is a thin layer of colloidal residues and some large flocs on the wall and the bottom of the tank in many wastewater treatment plants. No matter how you stir it and how long it takes, these residues are hard to dissolve, and it will often cause unnecessary trouble if they are not properly treated.

Polyacrylamide is an organic polymer flocculant water treatment, the molecular weight is relatively large, generally in the millions of levels. When dissolve Polyacrylamide, it swells first, and then dissolves slowly. If the amount of polyacrylamide added to water in one time is large and cannot be added evenly and slowly, the polyacrylamide that touches water first begins to swell, the surface area becomes larger, and then envelops the part that does not touch the water, thus forming some insoluble polyacrylamide groups. At present, most wastewater treatment plants generally use manual dosing. It is suggested that the water should be stirred up first, and then dosing slowly and evenly distributed above the water column through the inlet. It can reduce the probability of the caking problem, when the potion is washed into the water along flow direction, and to avoid contact with the inside of the tank as much as possible.
It is suggested that the automatic continuous feeding system should be adopted as far as possible when conditions permit, which not only saves manpower and improves efficiency, but also avoids caking as much as possible When you add PAM. In addition, the quality of polyacrylamide on the market is uneven at present. Some products have quality defects, such as poor hydrophilicity, insoluble substances, poor solubility and so on, which tend to agglomerate when adding PAM.

Marketing Center: Room 603, RuiYing Building, Chengnan 1st Road, Dali Town, Nanhai District, Foshan City, Guangdong Province,China, 528231
Factory: Area A No.9, Technology Park, Sanshui District, Foshan, Guangdong, China
Tel : +86 13929123248
Email : exp006@broviecn.com
Fax : 0086-0757-85510580
Website : http://www.bv-chem.com