In
biochemical experiments, buffer solution plays an indispensable role,
it can resist the influence of a small amount of strong acid and alkali
and maintain the pH value closest to the physiological environment for
the system. HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid)
buffer and PIPES buffer are both commonly used in biological
experiments, both of which are Good's buffers and have similar
structures. Many people often have doubts: Besides the structure, what
is the difference between them?
Similarities between HEPES and PIPES buffers
HEPES and PIPES buffers, even all Good's buffers, have the following characteristics:
(1) pKa value between 6.0 and 8.0;
(2) High solubility in water;
(3) Membrane impermeability and not easy to penetrate biofilm;
(4) Limited impact on biochemical reactions, chemical and enzymatic hydrolysis, and no complex or precipitation with metal ions;
(5) Very low absorption of visible light and ultraviolet light;
(6) Ion concentration, solution composition and temperature have little effect on dissociation;
(7) Not participate or interfere with biochemical processes
What is the difference between them?
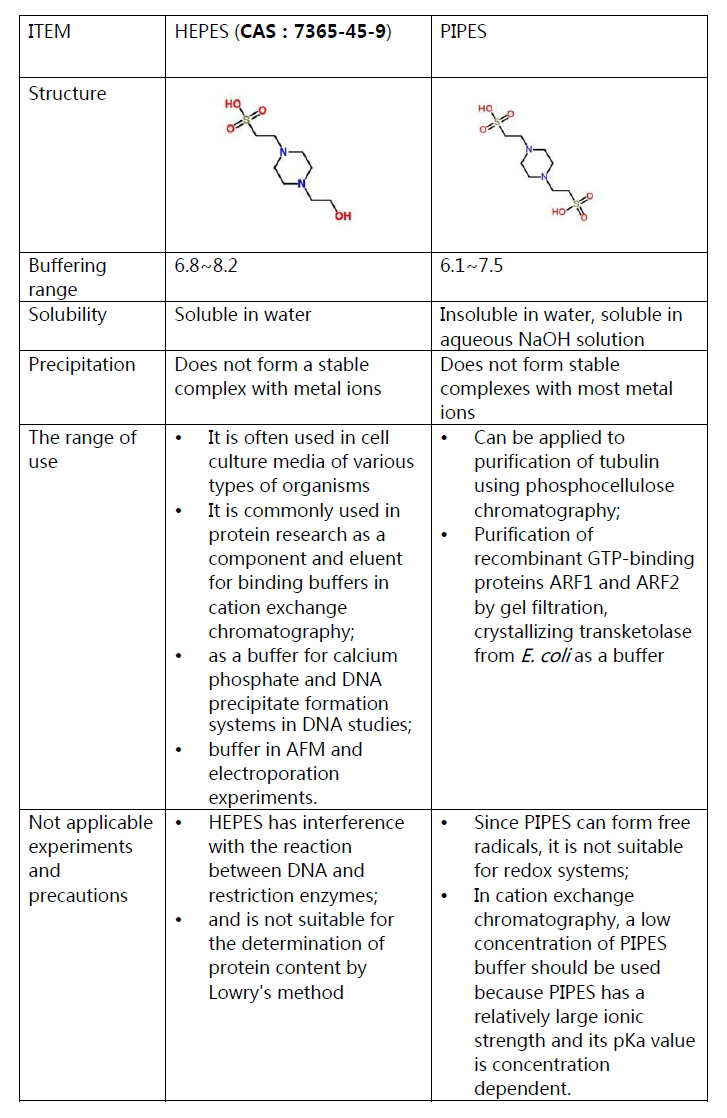
In summary, both HEPES buffer (CAS 7365-45-9)
and PIPES are Good’s buffers, which do not form stable complexes with
metal ions and are suitable for solution systems containing metal ions.
However, there is also a certain difference between them. Therefore,
when selecting the above buffer, we need to comprehensively consider the
suitability of the experimental system and the difference in the nature
of the two.
Edited by Suzhou Yacoo Science Co., Ltd.
没有评论:
发表评论