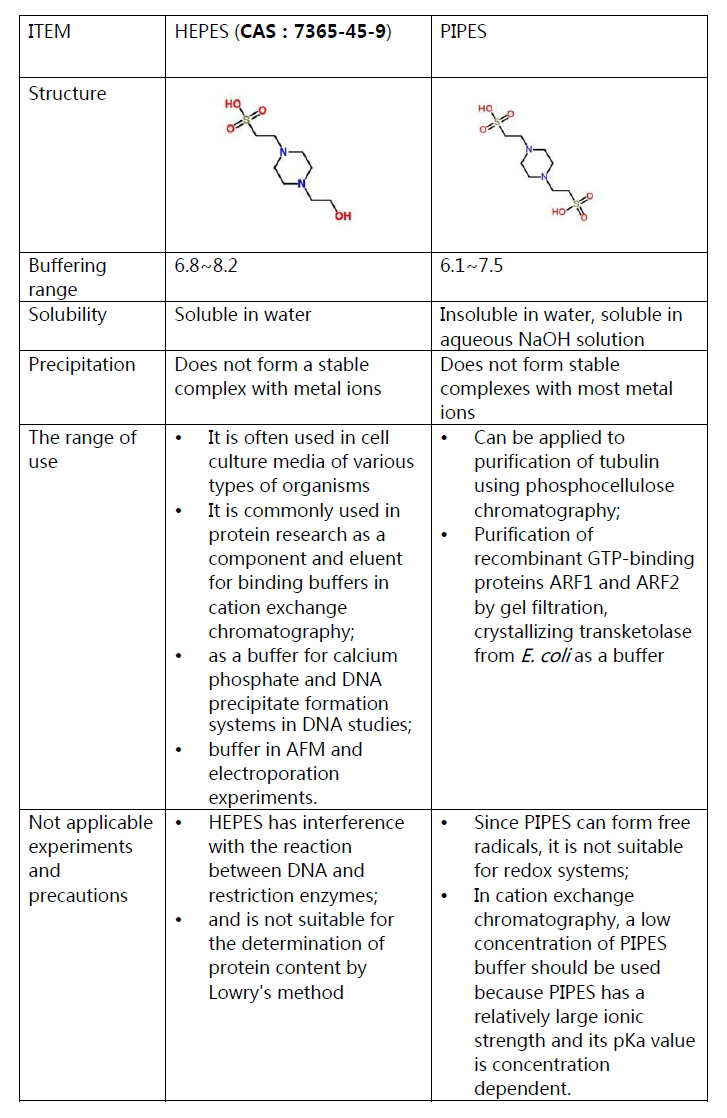
HEPES is abbreviated of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid,
which is a Good's buffer with pH buffer range of 6.8~8.2, and widely
used in cell culture because of its good ability at maintaining
physiological pH. It can also be used to prepare porous zinc oxide
microspheres.
As
a II-VI semiconductor compound, zinc oxide has a band gap of 3.37 eV
and an exciton binding energy of 60 meV. Due to its special
piezoelectric and optical properties, zinc oxide is widely used in many
fields such as solar cells, sensors, and voltage sensitive resistors,
piezoelectric materials, antibacterial agents, and photocatalysis
fields. The morphology and size of zinc oxide have an important
influence on its properties and applications, especially porous zinc
oxide microspheres, which exhibit low density and high specific surface
properties in catalysts, gas sensors, drug delivery and other fields.
At
present, different methods have been reported to synthesize porous zinc
oxide microspheres, such as high temperature calcination, chemical
vapor deposition, and chemical bath deposition. However, these methods
have high reaction temperatures, are complicated to operate, and are
difficult to control. The solvothermal method is widely used for the
synthesis of zinc oxide micro/nano materials due to its simple equipment
and mild reaction conditions. However, the synthesis of porous zinc
oxide microspheres by solvothermal method often requires the addition of
a templating agent or a porogen. After the completion of the reaction,
further post-treatment is required to remove the templating agent, which
increases the complexity of the process and environmental pollution.
In
order to solve the above problems, the researchers [1] developed a
preparation method for porous zinc oxide microspheres by using HEPES
molecules, which has low cost and simple operation, and the obtained
zinc oxide has uniform morphology, high specific surface area and
multi-stage pore structure. The specific operations are as follows:
(1)
2~6 mmol of organic zinc salt (zinc acetate or zinc acetylacetonate)
are sonicated in 30~70mL organic solvent (TEG, DEG, EG, DMF) for 10
minutes, then add 2~8 mmol HEPES;
(2)
The mixed solution is placed in a stainless steel high pressure
autoclave lined with 100 mL of polytetrafluoroethylene, and reacted at
150°C for 6 to 18 hours;
(3)
The obtained product was washed by centrifugation at 10000 rpm/min for
10 minutes, and the supernatant was removed, repeat 5 times to remove
the residual solvent and HEPES. The product was dried in a 60°C oven for
24 hours and then naturally cooled.
In
this method, HEPES molecules play an important role in the formation of
porous zinc oxide microspheres. After the addition of the HEPES
molecule, it is adsorbed on the surface of the nucleus by electrostatic
interaction with the zinc oxide seed crystal in the sol. The presence of
the HEPES molecule blocks further clustering and aggregation of the
nucleus. Porous zinc oxide microspheres are formed by the continued
growth of the HEPES molecules under solvothermal conditions. The HEPES
molecules and solvent in the surface and voids of the microspheres can
be completely removed after multiple washings with deionized water.
HEPES (CAS 7365-45-9)
molecules have the advantages of non-toxicity and environmental
friendliness. The method is easy to operate, simple in equipment, low in
synthesis temperature, low price in raw materials, good in
repeatability, and suitable for industrial production. The prepared
porous zinc oxide microsphere has the advantages of uniform size,
multi-stage pore structure (pore size 4~30 nm) and large specific
surface area (43.4~69.6m2/g), and can be used as photocatalyst and gas
sensor.
Reference
[1]CHEN
Rong, LI Qin, YANG Hao, Lv Zhong. A preparation method of porous zinc
oxide microspheres guided by HEPES molecularly. 2014, CN103482682A.
Edited by Suzhou Yacoo Science Co., Ltd.